An acute myocardial infarction (AMI), or heart attack, is a life-threatening condition that occurs when blood flow to the heart muscle (myocardium) is suddenly decreased or completely cut off. It is usually caused by a blood clot or blockage in one or more of the coronary vessels supplying blood to the heart muscle. An AMI requires immediate treatment and medical attention, as any delay in intervention can result in irreversible damage to the heart muscle. According to the Centers for Disease Control and Prevention, each year more than 800,000 people living in the US will suffer a heart attack.
Although the management of AMI has improved in recent decades, morbidity and mortality associated with AMI remain high, with the majority of early deaths occurring outside the hospital. Early action is crucial for survival and to preserve heart muscle. Besides aspirin, there are no treatment options currently available for the critical time from onset of AMI symptoms to first medical contact. The need for an early intervention has been highlighted by the guidelines of the European Society of Cardiology, which identified the prehospital phase as the most critical for high-risk patients and reiterated that efforts must be made to reduce the delay in initiation of treatment in order to reduce deaths.
Dual antiplatelet therapy – the combination of aspirin and a P2Y12 inhibitor – is a cornerstone of the treatment of patients with acute coronary syndromes (ACS) and of those undergoing percutaneous coronary intervention (PCI). Oral P2Y12 inhibitors are indicated for acute treatment as well as long-term secondary prophylaxis of confirmed AMI. An intravenous P2Y12 inhibitor is intended for specialized use in an acute and hospital setting in patients undergoing PCI who have not been pretreated with an oral P2Y12 inhibitor.
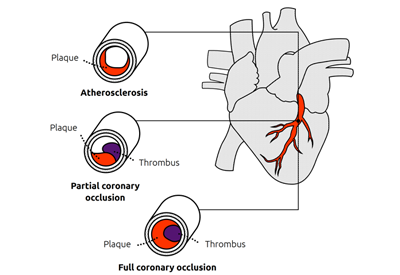
Platelet adhesion, activation, and aggregation play a pivotal role in atherothrombosis. An essential element in the platelet activation process is the interaction of adenosine diphosphate (ADP) with the platelet P2Y12 receptor. This platelet activation and aggregation can be inhibited by antagonizing the platelet P2Y12 receptor. This prevents the binding of ADP to the receptor, which reduces platelet aggregation and the reaction of platelets to stimuli of thrombus aggregation.
P2Y12 inhibitors have been used in the treatment of millions of patients globally, and the safety and efficacy profiles are well established. However, until now, the method of administration or the delayed onset of effect means that currently available treatments do not have the desired profile to cover the critical time from onset of AMI symptoms to first medical contact.
Selatogrel is a potent, fast-acting, reversible, and highly selective P2Y12 inhibitor, being developed for the treatment of AMI in patients with a recent history of AMI. It is intended to be self-administered subcutaneously via a drug delivery system (autoinjector). This novel, self-administered emergency agent has the potential to protect heart muscle in the very early phase of an AMI – in the crucial time between symptom onset and first medical attention – so as to treat the ongoing AMI and prevent early death.
Two Phase 2 studies in patients with chronic coronary syndromes and AMI, respectively, met their pharmacodynamic objectives of significantly inhibiting platelet aggregation. Subcutaneous administration of selatogrel 8 mg and 16 mg demonstrated a rapid onset of action, within 15 minutes, with the height of its effect extending over four to eight hours, depending on the dose. Selatogrel was safe and well tolerated in both studies, and there were no treatment-emergent serious bleeds.
In late 2019, Idorsia entered into a global development agreement with Halozyme, to design and customize an autoinjector for subcutaneous delivery of selatogrel. Halozyme’s autoinjector was selected for its robustness, reliability, ease-of-use, and emergency-ready capabilities – key requirements due to the nature of AMI. Idorsia validated the usability of Halozyme’s autoinjector through human factor validation studies prior to initiating Phase 3 investigation.

Current status
Idorsia is enrolling patients into a large international, double-blind, randomized, placebo-controlled Phase 3 study – Selatogrel Outcome Study in suspected Acute Myocardial Infarction (SOS-AMI) – to assess the clinical efficacy and safety of selatogrel 16 mg when self administered (on top of standard of care) upon the occurrence of symptoms suggestive of AMI. The primary efficacy endpoint is the occurrence of death from any cause, or non-fatal AMI, after self-administration of the study treatment.
A Special Protocol Assessment has been agreed with the FDA, indicating its concurrence with the adequacy and acceptability of critical elements of overall protocol design for a study intended to support a future marketing application. In addition, the FDA designated the investigation of selatogrel for the treatment of suspected AMI as a “fast-track” development program. This designation is intended to promote communication and collaboration between the FDA and pharmaceutical companies for drugs that treat serious conditions and fill an unmet medical need.
SOS-AMI has been designed as a patient-centric study, with patients playing a key role. Patients participating in the study will be trained by qualified professionals, appointed at each study site, on how to recognize AMI symptoms and how and where to self-inject treatment; they will also be instructed to call for emergency medical help immediately. Trainers will use standardized material, mirrored across all countries, which has been developed with the support of education experts, with feedback from post-MI patients, and in compliance with current guidelines.
The Phase 3 study with selatogrel is currently recruiting patients, with a target enrollment of approximately 14,000 patients at risk of recurrent acute myocardial infarction.
Milestones
2021 Initiation of Phase 3 registration study
2019 Drug-device development agreement with Halozyme
2018 Positive results for the Phase 2 studies
Key scientific literature
-
Benjamin EJ, et al. Heart Disease and Stroke Statistics—2019;139(10):e56-e528.
-
Adnet F, et al. Emerg Med J. 2011;28(10):884–6.
-
Norris RM. BMJ 1998;316(7137):1065–70.
-
Ibanez B, et al. 2017. Eur Heart J 2018;39(2):119–77
-
Neumann FJ, et al. 2018. Eur Heart J 2019;40(2):87–165
-
Storey R. F, et al. Eur Heart J 2019;0, 1-9, doi:10.1093/eurheartj/ehz807
-
Sinnaeve P, et al. J Am Coll Cardiol. 2020 May 26;75(20):2588-2597. doi:0.1016/j.jacc.2020.03.059. PMID: 32439008.
-
Juif P, et al. The Journal of Clinical Pharmacology 2019, 59(1): 123-130. doi:10.1002/jcph.1296